
NCH-41

J H. Bischoff, South Dakota State University J.D. Oster, University of California (Riverside) D.E. Eisenhauer, University of Nebraska R.W. Shawcroft, Central/Great Plains Research Cent., CO G.V. Johnson, Oklahoma State University D.A. Whitney, Kansas State University
Use of "poor quality" waler to irrigate corn can lead to reduction in crop yield, deterioration of the soil structure or even plant uptake of toxic substances. The major factors determining the quality of irrigation water are its salt content (salinity); its salt composition (sodicity); its sediment load; and type and amount of toxic constituents, including pathogens and pesticide residues.
All surface and ground waters contain dissolved salts, and many surface waters are degraded by sediment. Irrigation return flows may contain pesticide residues, excessive nutrients, and, in some cases, pathogens. Increased reuse of water supplies for irrigation, including sewage effluent, increases the potential for water quality problems.
Rarely are all water quality factors of concern in any given field at any one time. But when present, each must be properly managed to prevent yield loss or contamination of our soil and water resources. With appropriate preventative measures and sound water management, potential adverse effects from irrigating with water of marginal quality can be avoided.
Water quality must be evaluated in terms of intended use. Irrigation water having certain properties that would be desirable, or at least acceptable, in one instance may cause problems when used for another purpose. Thus, proper evaluation of quality can be made only when applied to a specific set of conditions. The information presented in this publication is specifically for corn production, and some of it may not be applicable to other crops. Also, some of the quality criteria may have to be adjusted for conditions unique to a particular site.
Salinity is determined by the total amount of dissolved solids (salts) present in a given volume of irrigation water. It affects crop growth by creating an osmotic stress that restricts water uptake and evapotranspiration.
Irrigation water salinity is frequently estimated by measuring the water's electrical conductivity (ECiw) in decisiemens per meter (dS/m) instead of its total dissolved solids in parts per million (ppm), simply because ECiw is easier to determine (see sidebar on next page regarding measures of water quality). Total dissolved solids can be estimated by multiplying ECiw by 640 (although the exact relationship may vary somewhat with the kind of salts present).
Electrical conductivity of soil water (ECsw) can be determined using sensors buried in the root zone. Electrical conductivity of a saturation extract (ECe)-a common laboratory measurement of salinity from soil samples-is usually about one half the ECsw value.
The typical cations in irrigation water are calcium, magnesium, sodium, and potassium; typical anions are bicarbonate, sulfate, and chloride (Table 1). Silicate may also be present but is seldom measured.
Other likely constituents include nitrate, carbonate, phosphate, and trace elements, which are usually present in low concentrations in natural waters. Nitrate levels can be significant in certain localized areas. Although beneficial to crop production, nitrate does pose a human health problem if present in domestic water supplies at concentrations above 10 ppm of N. Except for boron in a few local areas, trace elements are seldom high enough in irrigation waters to be toxic to corn.
To be useful, any irrigation water salinity assessment must be made in view of both the salt tolerance and the leaching requirement of the intended crop.
The measures of water quality used here are in units commonly used by irrigators, extension personnel, and testing laboratories. Because scientists may express the same measures in different units (usually metric), following is a series of common equivalences:
* Electrical conductivity in millimhos per centimeter (mmho/cm) is numerically equivalent to conductivity in decisiemens per meter (dS/m).
* Concentration of total dissolved solids in parts per million (ppm) is numerically equivalent to concentration in milligrams per liter (mg/I) or grams per cubic meter (g/m3 ).
* Concentration of an ion in solution in millimoles per liter (mmol/l) or moles per cubic meter (mol/m3) is equal to concentration in mg/I divided by the ionic weight.
* Concentration of an individual ion in milliequivalents per liter (meq/l) is numerically equivalent to the valence of the ion times its concentration in mol/m3.
* Sodium adsorption ratio (SAP) is frequently given the symbol RNa; although usually expressed without dimensions, units of (meq/l)1/2 are implied.
Chemists have traditionally expressed concentration of individual ions in milliequivalents per liter (meq/I) to permit comparisons on a chemically equivalent basis. Total salt concentration is the sum of concentrations of all ions present. These must be expressed in consistent units, whether ppm mg/I, meq/I, etc. Symbols and units of measurements are shown in Table 1.
Unit of Atomic
Determination Symbol Valence measure weight
---------------------------------------------------
Constituents
Cations
calcium Ca +2 meg/I 40.1
magnesium Mg +2 meq/I 24.3
sodium Na +1 meq/I 23.0
potassium K +1 meq/I 39.1
Anions
bicarbonate HCO3 -1 meq/I 61.0
sulfate SO4 -2 meq/I 96.1
chloride Cl -1 meq/I 35.5
Trace elements
boron B ppm 10.8
Total Salt content
Electrical conduc- ECiw - dS/m -
tivity
Concentration C - ppm -
Sodium hazard
Sodium adsorption SAR - (meq/I)1/2 -
ratio
--------------------------------------------------
Corn salt tolerance. The degree to which a crop's productivity will be affected by soil salinity is termed its salt tolerance. Crops differ widely in salt tolerance; consequently, irrigation water salinity may dictate what crops can or should be grown.
Corn is moderately sensitive to salinity, being more sensitive than most field crops and about equal to many vegetable crops. With proper management, however, corn can be grown over a fairly wide range of irrigation water qualities, particularly where rainfall is sufficient to dilute or leach saline soil water caused by irrigation applications.
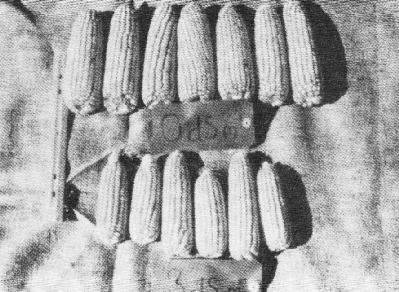
Symptoms of moderate salt stress in corn are difficult to observe in the field because the plants appear normal; but they will be somewhat stunted (Figure 1).
If electrical conductivity of soil water (ECsw) averages less than 3.7 dS/m (2400 ppm) through the growing season, grain yield should not be affected (1). However, if ECsw exceeds that 3.7 threshold level (or ECe exceeds 1.7), yield will be reduced 6 percent for each increase of 1 dS/m on mineral soils and 14 percent for each 1 dS/m increase on organic soils (Figure 2).
Corn grown for silage has a salt tolerance threshold similar to that grown for grain (ECsw 3.9 dS/m or ECe = 1.8). Stover production, however, is reduced much more rapidly than grain production at salinity values higher than the threshold-about 14 percent for each 1 dS/m increase on both mineral and organic soils.
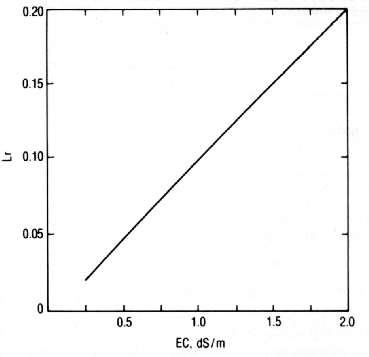
Leaching requirement. Following an irrigation, the soil's concentration of soluble salt increases as the water is removed by evaporation and transpiration. If there is little or no leaching (i.e., movement of water down through the soil root zone), salt accumulates in the soil with successive irrigations. If leaching occurs, soluble salt accumulation is "controlled" by the proportion of applied water that drains below the root zone (the leaching fraction). The amount of salt that will precipitate is a further consideration if the soil leaching fraction is less than about 0.1.
The leaching fraction that must be maintained to prevent loss of crop productivity is the leaching requirement (Lr). Figure 3 allows you to determine the Lr for water of various salinity levels in order to maintain corn productivity.
Where it is not essential to meet the leaching requirement during the growing season, the salts may be leached from the soil in winter or before planting. Off-season rainfall may also accomplish all or at least part of the leaching requirement.
Salinity and corn growth stage. Irrigation water salinity has its greatest influence on corn growth or yield response during the seedling stage, which is the crop's most critical growth period. At 3 weeks of age, the corn plant has a salt tolerance threshold of only 1 dS/m; whereas by the tasseling and grain-filling stages, it can tolerate an ECsw up to 9 dS/m without yield loss. Thus, any irrigation practice that results in excessive soil salinity between germination and tasseling should be avoided.


The type and amount of dissolved solids present in irrigation waters vary significantly, depending on such diverse factors as supply origin, geographic location, and climatic and seasonal variations.
Surface waters. The average salt content of rivers and streams is estimated at about 120 ppm, but the range is large. Rivers in arid regions tend to contain more salts than those in higher rainfall regions. Table 2 shows the salinity levels of six representative river waters in the United States. The values are year-round averages; concentrations may vary widely throughout the year and usually in inverse relation to stream flow.
Seasonal variation and weather events can also affect surface water salinity. For example, streams originating in mountain regions usually have low salt content but high amounts of suspended sediment during May and June because they consist mostly of snowmelt. Later, as snowmelt runoff declines, ground water base flows to these streams increase their salt content. Summer storms in the watershed can cause temporary increases in the sediment load along with corresponding decreases in salinity.
Surface water salinity is usually lowest near the stream source. As highly mineralized ground water enters a river or stream and/or it is repeatedly used (especially for irrigation), the water becomes more and more saline.
Ground waters. Ground waters are often more saline and contain higher proportions of sodium, boron, and nitrate than surface waters. Ground water quality is rarely influenced by climate, season or even pumping duration; but it is likely to differ from one well to another, reflecting the composition of different strata from which water is being pumped. Salt content could increase with pumping duration where dense, salty water exists in the lower part of an aquifer. Table 2 shows the salinity levels of irrigation wells at eight locations throughout the U.S.
Salinity Sodicity
-------- ----------
Electrical Sodium
conduc- Salt adsorption
tivity content ratio
Water source and location In dS/m in ppm in (meq/I)1/2
-----------------------------------------------------------
River waters
SAN JOAQUIN at Friant 0.04 20 0.4
Dam, CA
SNAKE at King Hill, ID 0.5 370 0.9
SOUTH BRANCH at Paw 0.56 359 0.35
Paw River, MI
PLATTE at Brady, NE 0.7 503 1.6
COLORADO at Grand Junc- 1.05 623 3.3
tion, CO
PECOS at Artesia, NM 8.6 6010 12.5
Well water
MILLER Co., GA* 0.26 149 0.06
RIVERSIDE Co. at Indio, CA 0.3 230 1.4
ADAMS Co. at Hastings, NE 0.5 370 0.9
VAN BUREN Co., MI* 0.52 333 0.37
SCOTT Co., KS 0.73 450 0.9
KERN Co. at Bakersfield, CA 0.8 530 23.1
REEVES Co. at Pecos, Tx 4.4 2860 6.1
SALINE Co., NE 7.4 4310 49.0
------------------------------------------------------------
*Unpublished data provided by T. A. Cummings, USGS District Chief.
Lansing. Ml, and J. B. Mcconnell, USGS chemist. Doraville, GA.
Corn is commonly irrigated by furrow or sprinkler methods, although trickle irrigation is also being used. The methods differ in their effects on salinity in the root zone. For instance:
* With surface methods like furrow irrigation, quantities of water are applied infrequently, and the crop then utilizes what water is stored in the root zone between irrigations. As the water is used, that which remains increases in salt content. Thus, more frequent irrigations may be necessary to control salinity it saline water is being applied. However, some surface systems may lack the flexibility to permit frequent waterings.
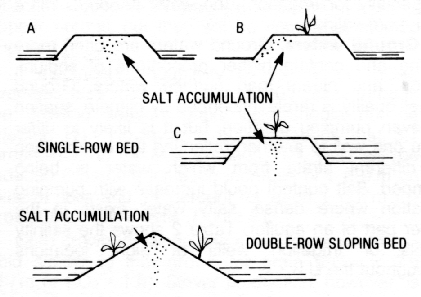
* Also with furrow irrigation, salt tends to accumulate in the ridges or beds. Soluble salts move with the soil water but are left behind as that water evaporates, making salt concentration higher in the center of the bed than on its slopes or edges (Figure 4). Consequently, corn should not be planted into the center of the bed if salinity is a potential hazard.
* With sprinkler irrigation, saline water applied under high evaporative conditions could cause injury to corn leaves. Margin- or tip-burn has shown up in corn sprinkled during the day with water having an ECiw greater than 4 dS/m. Plant growth would also likely be restricted under those conditions.
Conservation tillage practices, designed to maintain residues on the soil surface for more effective wind and water erosion control, are now quite common. Although still rather limited, research done so far indicates that conservation tillage does not require different salinity management practices or guidelines.
Some differences in salt movement and distribution should be expected under conservation tillage due to cooler soil temperatures and less evaporation from the soil surface. Leaching requirements may be easier to meet because of improved infiltration and less evaporation.
When calcium and magnesium are the predominant exchangeable cations, soil tends to have a granular structure that is easily tilled and readily permeable. As the amount of sodium adsorbed on the soil particles increases in relation to the calcium and magnesium for a given soil salinity level, the mineral particles tend to disperse, plugging pores and thus decreasing the soil's permeability to water.
Soil salinity reduces the effect of sodium. That means, with increasing salinity, increasing levels of sodium are tolerable. When those levels become excessive, either the infiltration rate is reduced to where the crop cannot get enough water or the soil profile hydraulic conductivity is reduced to where drainage is inadequate. Excessive sodium also adds to cropping difficulties because of seedbed crusting, temporary saturation of the surface soil, and/or possible disease, weed, oxygen, nutritional, and salinity problems.
The sodium adsorption ratio (SAP) of irrigation water is generally a good indicator of the exchangeable sodium status that will occur in the soil. In its simplest form, SAP can be calculated using the following equation, if the concentration (C) of all cations are expressed in units of meq/I (see Table 1):
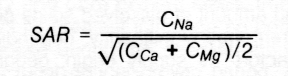
Soil permeability should not be a problem where irrigation water SAP values are below 10 (meq/I)1/2, provided the water is not too low in salt content (i.e., less than 0.3 dS/m). On the other hand, permeability problems are likely where SAP values consistently run above 10. For example, the Kern Co. (CA) well water with an SAP of 23.1 (Table 2) would be expected to cause permeability problems. In some cases, irrigation water SAP must be adjusted to account for the influence of carbonates and bicarbonates on calcium and magnesium precipitation.
As just noted, irrigation water with less than 0.3 dS/m salt content can adversely affect soil permeability, even when the SAP is less than 10. Such dilute water not only allows maximum swelling and dispersion of both soil minerals and organic matter, but also has tremendous capacity to dissolve and remove calcium, thus affecting infiltration.
One example is the water from upper reaches of the San Joaquin River in California (see Table 2), which may cause permeability problems on certain soils even though the SAP is only 0.4. In fact, in parts of the San Joaquin Valley, adding gypsum to irrigation water is a common practice because of its extremely low salt content.
Sediment can reduce the infiltration rate of a soil and can interfere with the operation of an irrigation system. Unless water velocities are very low, fine sediments remain in suspension to be carried through the system and discharged onto the field. Larger particles settle out easily to reduce channel capacity where deposited, which is frequently upstream of control structures.
Suspended sediments in irrigation water can, over time, affect soil permeability. As the water infiltrates, the sediments are filtered out, reducing the porosity, thus infiltration, of the surface layer.
For soils that are moderately to highly permeable, this is often regarded as a benefit, because the reduced infiltration rate allows longer irrigation runs or faster field coverage than would otherwise be possible. For soils that are initially fine-textured, however, suspended sediments in the irrigation water may simply further lower an already low infiltration rate.
There are few remedies for this problem, the main one being to remove suspended sediments before they reach the field. Another would be cultivating furrows between irrigations to reduce the effect of sediment accumulation.
With a pressurized system, sediment can abrade pump impellers and water outlets such as sprinkler nozzles. If sediment is present, pump efficiencies should be checked periodically by first calculating water horsepower from pump discharge rate and pressure measurements, then comparing horsepower with the rate of energy consumption. A significant decline in efficiency with time signals possible pump damage by sediment. (Many extension services, utility companies, and private consultants can provide assistance in checking pump efficiencies.)
Abrasion of sprinkler nozzles can be determined either by measuring the diameter of the nozzle bore or by comparing water distribution patterns of sprinklers with old and new nozzles.
With a trickle system, small amounts of sediment (even chemicals and bacteria) can clog many types of emitters. Filter systems are available commercially for treating the water supply and should be used unless the water is known to be sediment-free.
Criteria for evaluating the quality of water supplies for trickle irrigation, degree of hazard that each supply represents, and appropriate treatment measures are discussed by Gilbert et al. in a publication of the American Society of Agricultural Engineers (2).
Adverse effects of sediment either to the soil or to an irrigation system can often be minimized through one or more of the following management practices:
* Providing a settling basin or similar sediment-exclusion structure near the entrance of the conduit.
* Flushing the system with flowing water immediately following each irrigation before the sediments consolidate and dry.
* Sequencing irrigations of a field from upstream to downstream sets so that, at the end of the irrigation, water is flowing the entire length of the conduit. This procedure allows sediments to be flushed onto the last set or through a waste way at the end of the conduit.
Chloride and sodium, the solutes in saline water of concern for some crops, are not detrimental to corn except for their osmotic effects. Boron concentration in irrigation water above 1 ppm could cause boron toxicity symptoms. Plant pathogens and pesticide residues may also occur in toxic concentrations under some circumstances.
Pathogenic fungi or bacteria can be transmitted in recycled tail water or sewage effluent and/or can be spread through a field from one irrigation set to the next. For instance, corn fields showing bacterial wilt symptoms were found to have been irrigated with reuse (return-flow) water containing the bacteria.
Chlorination of reuse water can remove pathogenic bacteria but is less effective against fungal pathogens to a point that it may be impractical. If bacterial or fungal disease symptoms appear or conditions conducive to these diseases exist, tail waters should be wasted or only reused on nonsusceptible crops.
Ground water or rivers seldom contain pesticide residues in sufficient concentration to restrict their use for irrigating corn, However, if surface runoff from irrigated fields is salvaged and recycled onto adjacent fields or if water is transmitted through conveyances that have carried or been treated with pesticides, then residue concentration could possibly pose a hazard, Tail water from corn fields recently treated with herbicides should be reapplied only to the same or another corn field, not to a different crop,
If irrigating pesticide-treated corn fields or applying pesticides through the irrigation water, the potential for ground water contamination will be minimized (a) by applying no more water than is necessary to meet crop needs and (b) by ensuring that the mechanical equipment for injecting pesticides into irrigation water includes proper safeguards (3).
Runoff from surface-irrigated fields usually has a salt content similar to that of the initial irrigation water supply. If salinity of the original supply was suitable for corn, the runoff water should also be suitable.
Where surface irrigation has caused soil erosion, return flows could contain significant amounts of sediment. Suitability of the water must then be determined as discussed in the section on sediment. The phosphorus and/or pesticide concentrations may be greater than in the original supply if these chemicals were adsorbed on the eroded sediment particles.
Surface return flows from furrow-irrigated corn fields may contain large amounts of crop residue, especially during the pollination period. If so, some mechanism must be provided for screening or skimming off this floating material so it cannot enter the reuse pump intake.
Subsurface return flows are sometimes mixed with naturally occurring ground water. Because quality of such drainage water can be highly variable, its suitability for corn irrigation should be determined as discussed in the sections on salinity and sodicity.
Use of municipal sewage effluent for corn irrigation can benefit both the sewage treatment plant and the corn producer. Land application effectively removes nutrients, pathogens, and organic constituents from secondary-treated effluent, which is what the sewage plant wants. The effluent, in turn, provides many essential plant nutrients and improves soil tilth from the organic matter, which is what the producer wants.
Secondary-treated effluent generally has low concentrations of suspended solids, thus can be applied by most irrigation methods. In some communities in western states, water supplies owned by irrigation districts are first made available to municipalities, which use them and then make the treated effluent available for irrigation. With proper management, treated effluent has proven to be an acceptable long-term irrigation water supply.
Secondary sewage effluents may contain up to 250 pounds of nitrogen per acre-foot of water. This can provide much of a corn crop's nitrogen requirement. If effluent is to be used, its nutrient content should be analyzed and one's fertilization program adjusted both to avoid pollution from excess fertilizer applications and to take full advantage of potential fertilizer cost savings. For example, in 1975, 5,400 acres of corn fertilized with effluent from the Muskegon Co. (MI) sewage treatment plant received nutrients worth over $100,000.
Safe use of treated effluents. Before beginning to use treated municipal waste waters for irrigation (and periodically thereafter), samples should be taken and analyzed to insure that salinity, sodicity, and/or toxicity problems will not arise.
The presence of bacteria, viruses, and fungi in effluents is of concern with regard to the health of irrigators and the residues that may remain on harvested crops. Present data do not reveal extraordinary health problems among sewage plant workers exposed daily to the materials being treated. Thus, by not applying raw sewage and by avoiding aerosols from sprinklers, irrigators should be at low risk.
Secondary effluent can be used with relative safety to irrigate crops not intended for direct human consumption. Nonetheless, the irrigation system should be designed and operated such that the effluent will not run off fields or get into ground water either from the fields or from storage or tailwater ponds.
Sewage effluent may contain copper, zinc, cadmium, lead, nickel, cobalt, and other metals. Their amounts and distribution will vary greatly, depending on the types of industry discharging waste into the sewage system. Corn can absorb these metals and conceivably accumulate levels that would be of concern when the plants are fed to man or animals.
However, most of the heavy metals in treated sewage are in the sludge, not the effluent. Furthermore, zinc, nickel, copper, lead, and cadmium are not very soluble when soil pH is greater than 6.5. (Acid soils can be limed to raise their pH.) Also, cereals such as corn usually accumulate less heavy metal from the soil than do vegetable crops, and that which is absorbed tends to remain in the roots, not in the grain.
If a corn crop does absorb high amounts of zinc, copper, cobalt, or nickel, it will be visually damaged at levels less than would be toxic to man or other mammals. In such a case, the crop can be discarded.
Irrigators who apply sewage effluent to corn need to remember that other crops will eventually be grown on that same land. Chromium and cadmium, although perhaps not harmful to corn, could be absorbed later by other crops at levels potentially toxic to mammals. The U.S. Environmental Protection Agency has published provisional guidelines on maximum allowable application rates of cadmium to agricultural soils and maximum cumulative application of several other heavy metals (4).
Ground waters may contain significant quantities of plant nutrients, either from natural sources or percolated from feedlots or fields--in some cases, enough to supply most of the corn's nutrient requirements. A nitrate concentration of 1 ppm in an acre-foot of water is equal to 0.61 pound of nitrogen, Nitrate-N concentrations higher than 30 ppm have been reported in ground water pumped for irrigation. Ground waters may also contain appreciable amounts of sulfur, magnesium, calcium, and boron.
Production of irrigated corn can adversely affect the quality of underlying ground waters. The main concern is with nitrate, which is highly soluble, thus easily leached below the crop root zone if excess irrigation or precipitation occurs. Other mobile nutrients may also be leached into ground waters.
Potential for affecting ground water quality is greatest where surface soils are coarse-textured and/or water tables are shallow. With coarse soils, infiltrating water can easily exceed the waterholding capacity of the root zone and percolate below it; also there is a lack of fine soil particles to adsorb the chemicals. With shallow water tables, the effects of percolating solutes show up sooner than with deep aquifers, because the shorter distance between root zone and water table means less chance for adsorption and degradation.
To minimize nitrate additions to ground waters, irrigations should not refill the root zone completely if there is a good chance of precipitation before the plants can use much of the nitrogen. Also, on soils subject to deep percolation, the N applications should be split up until the ear-filling stage of corn, rather than all made prior to planting. In general, irrigations that are managed to conserve water will minimize the chances for ground water pollution.
Currently there is little information on pollution of ground water by pesticides applied to irrigated corn. In one study, where corn received twice the normal irrigation amount and 10-20 times the normal insecticide application, the water in observation wells 2-20 meters deep contained no measurable residues. The conclusion was that insecticide applications at maximum recommended rates for 5 years had not contaminated the ground water.
However, the potential does exist for pesticide contamination of ground water, particularly when pesticides are water soluble and applied to highly permeable, coarse-textured soils, which are less able to adsorb the chemicals. For instance, appreciable levels of aldicarb have been found in ground waters in Wisconsin and New York; the same for DBCP in California.
Aluminum irrigation piping can be encrusted or corroded when water's salt content is high. Encrustation reduces pipe capacity, whereas corrosion can rapidly weaken the pipe walls and cause leaks. Plastic piping should be considered if the irrigation water's constituents would damage aluminum.
Fertilizer materials added to irrigation water, such as ammonium nitrate, phosphoric acid, and ammonium sulfate, can be very corrosive to the metal components of sprinkler systems. The phosphate attacks bronze and brass, especially in the presence of ammonium ions.
Highly concentrated fertilizer solutions in irrigation water are apt to be strongly acid or alkaline and may contain corrosive salts, such as potassium chloride. Most fertilizer solutions also attack galvanized steel to some extent. If any of these compounds are injected as fertilizers through the irrigation system, it should be flushed thoroughly with clean water at the end of each irrigating period.
Dissolved iron or manganese in the water supply of a trickle irrigation system may react with the chloride added for algae control to form insoluble precipitates that plug the trickle emitters. There are several effective solutions to this problem, the most practical being to use low levels of copper sulfate in lieu of chloride for algae control (5).
To insure that quality of the irrigation water supply is adequate for optimum corn production, it should be checked periodically. Most land-grant university and private testing laboratories will analyze samples for salinity, sodicity, and the more common toxic elements. Results of the analysis can then be checked against Table 3 to determine if the water supply will present production problems.
To be assured of a reliable analysis, the samples collected must (a) be representative of the water supply, (b) not be contaminated by the sampling procedure, and (C) arrive at the testing laboratory unchanged. Here are some suggestions for taking samples properly:
1. Surface water sources (lakes, streams, ponds) may be sampled any time during the irrigation season, but collect the sample several inches below the surface.
2. Ground water sources should be sampled during the peak of the irrigation season, with the wells pumped several hours before sampling.
3. Sample a test well only after a pipe and screen are installed and all water added during the drilling operation has been pumped out (at least 10 hours). The screen or sandpoint should be near the lower part of the water-bearing formation, since salt content is often highest at that level.
4. Collect at least a 1-pint sample in a clean plastic container. Rinse the container several times first with the water being sampled; and be certain the cap or lid is also clean, especially if using a detergent bottle. If the sample cannot be sent to the laboratory immediately after collection, refrigerate until it can be.
Potential for a problem
increas-
Type of irrigation problem None ing Severe
-----------------------------------------------------
SALINITY (affects crop water
availability)2
ECiw in dS/m <1.1 1.1-4 >4
SODICITY (affects soil perme-
ability to water)3
SAR in (meq/I)1/2
Montmorillonite soils4 <6 6-9 >9
illite-vermiculite soils4 <8 8-16 >16
Kaolinitic-sesquioxide <16 16-24 >24
soils4
SPECIFIC ION TOXICITY
ppm or mg/I
Boron <1 1-2 >2
Sodium -Corn is tolerant-
Chloride -Corn is tolerant-
Bicarbonate (overhead <400ppm ->400ppm-
sprinkler)
--------------------------------------------------------
1 Adapted from Ayers and Westcott (1976)
2 Management, rainfall, and growth stage modify salinity effects on
corn. See Figure 3 for determining leaching requirement.
3Potassium and ammonium ions also contribute to clay dispersion,
but somewhat higher levels are required than for sodium; the effects
can be additive, however. Significant amounts of ammonium ion may
occur in waste waters, but rarely in natural ground or surface
waters
4 Tolerance of soils to sodium saturation without affecting
permeability varies with type of clay in the soil. Montmorillonite
soils predominate in the Corn Belt, Great Plains, and western U.S.,
but illite-vermiculite soils are interspersed among them. Kaolinitic
soils predominate in the Southeast and occur in the Northeast, where
illite-vermiculite clays are most common. Use adjusted SAR for
calcareous soils.
If the water used for corn irrigation is of poor quality, one or more of the following practices may be necessary to avoid those soil problems that limit crop yields.
1. Provide adequate internal drainage. If barriers restrict movement of water through the root zone, water with either a moderate sodium hazard (SAP greater than 6) or salinity hazard (ECiw greater than 1.5) should not be used unless drainage can be provided.
2. Meet the necessary leaching requirement (over-irrigation) depending on crop and ECiw of water. A leaching requirement can be calculated from water test results and tolerance levels for specific crops. This is necessary to avoid buildup of salt in the soil solution to levels that will limit crop yields. Effective rainfall can be considered part of the leaching requirement.
3. Maintain higher available water in the soil. The soil should not be allowed to become more than moderately dry, since the crop cannot remove all the normally available water due to the higher salt content.
4. Monitor salt and sodium with saline-alkali soil tests every 1-2 years. Development of a sodium hazard usually takes time. Soil tests for SAR of saturation extract or percent exchangeable sodium can detect changes before permanent damage occurs. Proper management can maintain SAR and salinity values at a steady state below the danger level. Soil samples should be taken to represent the top foot and the second foot. Occasionally samples should be taken down to 4 feet.
5. Add soluble calcium such as gypsum (calcium sulfate) to decrease the SAR to a safe value. Gypsum can be metered into the water at the required rate, or in some cases it can be broadcast over the field annually. If broadcast, apply directly ahead of irrigation or thoroughly incorporate into the tillage layer to avoid crusting problems. If the soil contains free lime, elemental sulfur could be broadcast. The sulfur solubilizes the calcium from the free lime already in the soil. If gypsum is used, the leaching requirement may be increased. (Practice 2).
6. Restrict use of low-quality water. Apply it only during drought periods to supplement below-normal rainfall or when other sources of water are inadequate. The occasional use of Practice 4 may be necessary.
The "right" practice or combination of practices depends on which hazards are associated with the water you plan to use and the severity of those hazards. In fact, sometimes the risk of using poor-quality water may just be too great. Although Table 3 will help call attention to those risks, seek the advice of an expert if there is any question that your irrigation water supply poses a hazard.
New 1/87
Cooperative Extension Work in Agriculture and Home Economics, State of Indiana, Purdue University and U.S. Department of Agriculture Cooperating. H.A. Wadsworth, Director, West Lafayette, IN. Issued in furtherance of the Acts of May 8 and June 30, 1914. It is the policy of the Cooperative Extension Service of Purdue University that all persons shall have equal opportunity and access to our programs and facilities